What is a UF supercapacitor?
UF Supercapacitors, also known as electrochemical capacitors, electrical double layer capacitors, gold capacitors, and faraday capacitors, are electrochemical components developed from the 1970s and 1980s to store energy through polarized electrolytes. It is different from traditional chemical power sources and is a power source with special performance that lies between traditional capacitors and batteries. It mainly relies on double layers and redox pseudocapacitive charges to store electrical energy. But there is no chemical reaction during its energy storage process, which is reversible, and it is precisely because this supercapacitor can be repeatedly charged and discharged hundreds of thousands of times. Its basic principle, like other types of double-layer capacitors, is to use a double-layer structure composed of activated carbon porous electrodes and electrolytes to achieve ultra-high capacity. The outstanding advantages are high power density, short charging and discharging time, long cycle life, and wide operating temperature range, making it the largest capacity double layer capacitor in the world that has been put into mass production.
The feature of UF capacitors’s Supercapacitors /TSE Electric Double Layer Capacitors
70°C 1000 Hours Coin Type 5.5V
Long Cycle Life
Large Capacitance, Low ESR, High Monomer Consistency
RoHS, REACH Compliant

Identification of UF Supercapacitors and Batteries
Firstly, there is no electrochemical process during the charging and discharging process of supercapacitors based on the double layer principle. Therefore, the voltage of the supercapacitor can be released to zero, so the double layer supercapacitor is short circuited at both ends during storage, which means that the supercapacitor is not expected to carry charge or voltage when not in use. And the voltage of the battery is not allowed to be released to zero, nor is it allowed to short circuit the positive and negative electrodes, which will cause a short circuit and damage the battery. Therefore, it is easy to distinguish whether the two electrodes are short circuited in normal times. Of course, it is also effective to distinguish between supercapacitors and batteries by using whether they can be discharged to zero; Secondly, theoretically speaking, since the two electrodes of the supercapacitor are symmetrical, reverse voltage operation is allowed, while the battery is neither allowed nor possible; Thirdly, the relationship between the voltage and charge during the charging process of supercapacitors based on the double layer principle is linear, while the relationship between the voltage and charge of batteries is not linear.
If the identification between electrochemical supercapacitors and batteries is more difficult than the identification between supercapacitors and batteries based on the double layer principle. The reason is that the principle of electrochemical supercapacitors is the same as that of batteries, but the difference is that electrochemical supercapacitors also have a part of the double layer effect as part of energy storage. Without this, they do not belong to supercapacitors fundamentally; If you need to distinguish between electrochemical supercapacitors and batteries, you can start with energy density. The energy storage of electrochemical supercapacitors is much lower than that of batteries. Therefore, the comparison of discharge capacity can distinguish between electrochemical capacitors and batteries.
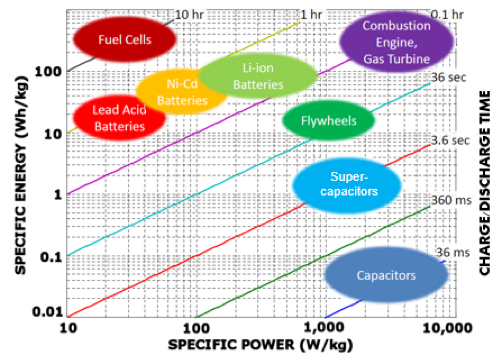
Power Density
Power density refers to the amount of time it takes a technology to charge and discharge energy. Batteries have the disadvantage in this characteristic due to the chemical reactions that take place to store and release energy. UF Supercapacitors have faster charge and discharge rates than batteries because the chemical reactions that take place within batteries take longer to release electrons than the electrical discharge in UF supercapacitors.
UF capacitorhas stock & fast delivery for it. See below advantage of super capacitors:
- Excellent Lead time 2-3 weeks or stock
- Stock items: Coin type super capacitors 0.22F, 0.33F, 0.47F, 0.68F, 1F 5.5V.
- Wide temperature: 70C/85C
- Cost down 30-60%